The water around proteins absorbs more terahertz radiation than pure water, which helps our proteins fold correctly.
If unfolded or misfolded proteins start to accumulate, the endoplasmic reticulum becomes stressed and activates a signaling pathway called the unfolded protein response.
The unfolded protein response is implicated in many diseases. For example, the response is often activated in rapidly growing cancer cells, which enables the cells to fold the large numbers of mutated proteins they produce. Also, viruses can trigger this response as part of their strategy to trick host cells into producing a number of difficult to fold viral proteins.
The correct folding of proteins is essential for cellular homeostasis and the prevention of disease. During times of stress or changes in demand for newly synthesized proteins, signaling pathways cooperate with networks of protein-folding and protein-degradation factors to maintain the pools of non-native proteins within a tolerable range. Such events are crucial for survival, as aberrant protein homeostasis can result in diseases that range from cancer and diabetes to neurodegeneration.
Understanding molecular vibrations in proteins are important because they regulate the function of proteins, e.g., in enzyme catalysis and protein interactions. In particular, low-frequency collective vibrational modes of proteins in the terahertz frequency region are expected to have a strong influence on protein function because terahertz radiation is absorbed by proteins.
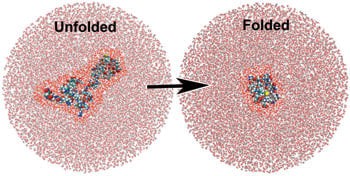
Image: A simulation showing water molecules around an unfolded and folded protein. The water molecules closest to the protein are highlighted bright red for effect. Terahertz spectroscopy has shown that the water molecules closest to the protein behave differently than the surrounding ones and actually participate in the folding process.

fenofibrate 160mg cost tricor 200mg generic fenofibrate online buy
cost ketotifen geodon without prescription tofranil 75mg brand
buy tadalafil 10mg without prescription order sildenafil pill viagra 50mg cheap
oral minoxytop mintop brand buy ed medication
buy acarbose 50mg generic fulvicin online order griseofulvin 250mg for sale
aspirin sale zovirax cream zovirax medication
order dipyridamole 100mg for sale felodipine pill pravastatin 10mg without prescription
order melatonin generic order aygestin generic capsules danazol 100mg
purchase duphaston without prescription order dapagliflozin 10 mg for sale order jardiance 10mg pills
Agora, a tecnologia de posicionamento tem sido amplamente utilizada. Muitos carros e telefones celulares têm funções de posicionamento e também existem muitos aplicativos de posicionamento. Quando seu telefone for perdido, você pode usar essas ferramentas para iniciar rapidamente as solicitações de rastreamento de localização. Entenda como localizar a localização do telefone, como localizar o telefone depois que ele for perdido?
Desde que haja uma rede, a gravação remota em tempo real pode ser realizada sem instalação de hardware especial.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Hello there, just became alert to your blog through Google, and found that it is really informative.
I am going to watch out for brussels. I’ll be grateful if
you continue this in future. Many people will be benefited from your writing.
Cheers!
Very interesting topic, thanks for posting.?
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me? https://accounts.binance.com/es-MX/register?ref=JHQQKNKN